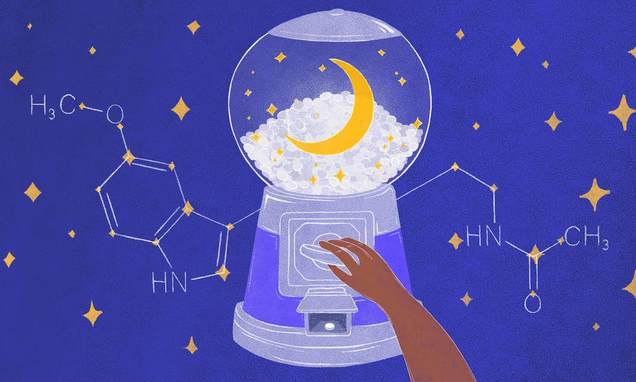
If you’re having trouble sleeping, melatonin is a popular and easy remedy. It’s effective for many people, doesn’t have any serious safety issues, and is available as pills or gummies for pennies a dose. It’s also misunderstood, though: melatonin is not a traditional sleeping pill.
Read more...

Huntsville AL-Qualitest Pharmaceuticals today issued a voluntary nationwide recall of all Accusure® Insulin Syringes. The distributed syringes are of the following descriptions and NDC numbers: 28G 1/2cc, NDC 0603-6995-21;28G 1cc, NDC 0603-6996-21; 29G 1/2cc NDC 0603-6997-21, 29G 1cc, NDC 0603-6998-21, 30G 1/2cc, NDC 0603-999-21, 30G 1cc, NDC 0603-7000-21, 31G 1/2cc, NDC 0603-7001-21, 31G 1cc, NDC 0603-7002-21. All Accusure® Insulin Syringes regardless of lot number are subject to this recall. These syringes were distributed between January 2002 and October 2009 to wholesale and retail pharmacies nationwide (including Puerto Rico). The syringes in these lots may have needles which detach from the syringe.
These products may contain undeclared whey (milk). People who have an allergy or severe sensitivity to whey (milk) run the risk of serious or life-threatening allergic reaction if they consume these products.
Pointe Scientific, Inc, Canton, MI is initiating a nationwide recall of all size kits of Liquid Glucose Hexokinase Reagent catalog number G7517. The reagents have been found to fail linearity at >200mg/dL that results in inaccurate glucose values above this range.
Mrs. Rios Corn Products in San Angelo, Texas is recalling its flour tortillas because they may contain undeclared Whey. People who have an allergy or severe sensitivity to Whey run the risk of serious or life-threatening allergic reaction if they consume these products.
Nationwide recall of all size kits. The reagents have been found to fail linearity at >200mg/dL that results in inaccurate glucose values above this range.
Cordis Corporation announced today a nationwide voluntary recall of all lots of the CROSSOVER™ Sheath Introducer due to complaints about stretching or fracture of the sheath during use.
The CROSSOVER™ Sheath Introducer is a product developed and manufactured by Thomas Medical Products, Inc., and distributed by Cordis. It is a long-coil reinforced, kink-resistant catheter sheath intended for use in arterial and venous procedures requiring the percutaneous, or through the skin, introduction of therapeutic or diagnostic intravascular devices or fluids
Today, Alexia Foods, Inc, Kennewick, WA, in cooperation with the U.S. Food and Drug Administration (FDA) is voluntarily recalling packages of its Alexia – Olive Oil, Sun-Dried Tomatoes & Pesto Oven Reds (frozen seasoned potato wedges) for an undeclared allergen, pine nuts. People who have an allergy or severe sensitivity to pine nuts run the risk of serious or life-threatening allergic reaction if they consume these products.
Pointe Scientific, Inc, Canton, MI is initiating a nationwide recall of all size kits of Liquid Glucose Hexokinase Reagent catalog number G7517. The reagents have been found to fail linearity at >200mg/dL that results in inaccurate glucose values above this range.
As part of its ongoing
cooperation with the Food and Drug Administration (“FDA"), Bodybuilding.com, LLC (the
“Company") announced today that it is conducting a voluntary nationwide and international
recall of all lots and expiration dates of 65 dietary supplement products (the “Recalled Products")
described on the attached list, that were sold through the Company’s website,
www.bodybuilding.com.
Morningstar Foods is voluntarily recalling 32-ounce Great Value Half & Half, UPC 6 05388 187 16 1, item code 1871600, plant code 21-031; 32-ounce Great Value 36% Heavy Whipping Cream, UPC 6 05388 187 18 5, item code 1871800, plant code 21-031; 32-ounce Kroger brand 36% Heavy Whipping Cream, UPC 0 11110 438 28 7, item code 4382900, plant code 21-031; and 64-ounce Wholesome Farms Chocolate Ice Cream Mix, UPC 0 74865 57 983 4 (if purchased in a multi-pack, the UPC code is 1 00 74865 57983 1), item code 5798300, plant code 21-031 because these products may contain soy protein.
American Regent conducts nationwide voluntary recall of ALL lots of its Ketorolac Tromethamine Injection, USP 15 mg/mL:
NDC# 0517-0601-25 15 mg/mL 1mL Single Dose Vial
PLEASE NOTE: This recall is in addition to the voluntary recall initiated on October 16, 2009 when American Regent voluntarily recalled ALL unexpired lots of Ketorolac Tromethamine Injection, USP, 30 mg/mL due to the presence of particulate matter in conjunction with crystallization.
Pelican Bay Ltd. of Dunedin, Florida is recalling all their Caramel Chocolate Truffle Hot Chocolate Mix because it may contain undeclared tree nuts. People who have an allergy or severe sensitivity to tree nuts run the risk of serious or life-threatening allergic reaction if they consume these products.
Kits recalled because the pediatric tracheal tubes were manufactured with an internal diameter smaller than indicated on the label.
Charleston Cookie Company of Charleston, SC is recalling packages of Almond Cookies which were sold as a component of the Dean and Deluca "Americana" cookie tin because the cookies contain undeclared butter (milk).
The U.S. Food and Drug Administration is issuing this health alert to warn consumers not to use Pig Ears and Beef Hooves pet treats manufactured by Pet Carousel because the products may be contaminated with Salmonella. The products were distributed nationwide in both bulk and retail packaging for sale in pet food and retail chain stores. Pet Carousel is based in Sanger, Calif.
PetSmart (NASDAQ: PETM) is voluntarily recalling two Dentley’s Beef Hoof products for potential salmonella contamination. The products were manufactured by Pet Carousel, Inc. in Sanger, Calif.
Hospira, Inc. (NYSE: HSP), a global specialty pharmaceutical and medication delivery company, is voluntarily recalling 85 lots of Liposyn™ II 10%, Liposyn II 20%, Liposyn III 10%, Liposyn III 20%, Liposyn III 30% and 73 lots of Propofol Injectable Emulsion 1% products that begin with the lot numbers 79 and 80 because some of the containers may contain particulate matter. The source of the particulate matter has been identified as stainless steel equipment used in the manufacturing process.
Jelly Belly Candy Company is recalling 7.5-ounce cylinder-style packages of 49 Flavors Jelly Belly jelly beans because the package is incorrectly labeled. The mislabeled packages failed to list peanut butter and peanut flour in the ingredient statement...
Reports of loss of device height, which may result in nerve injury, increased pain, spinal compression fracture, failure of additional fixation, and/or need for surgery to modify the implanted device.